FDA Approved Tepezza Label Update Now Provides Hearing Loss Warnings
A July 2023 Tepezza FDA label change adds important new warnings about hearing loss, and finally recommends that users undergo hearing testing before and after Tepezza treatment.
The FDA approved Tepezza only three years ago, but there has been growing evidence that the drug maker failed to adequately disclose all of the potential side effects at the time the thyroid eye disease drug was introduced. As a result, important Tepezza warnings were withheld from users and the medical community.
Our lawyers at Saiontz & Kirk, P.A. currently represent individuals throughout the U.S. who are now pursuing a Tepezza hearing loss lawsuit, alleging that they may have avoided permanent hearing damage if the Tepezza label had accurately disclosed risks associated with the thyroid eye disease treatment when the drug was first approved.
This month, the U.S. Food and Drug Administration (FDA) finally approved a Tepezza label update (PDF), which adds this important information to the “Warnings and Precautions” section, indicating that Tepezza may cause severe and permanent hearing loss. Doctors are now encouraged to test patients’ hearing before and after Tepezza treatments, and carefully consider the benefits and risks with users.
Unfortunately, these new warnings come too late for many former users, who are left with permanent Tepezza hearing loss side effects, including:
- Permanent hearing loss
- Partial hearing loss
- Tinnitus (ringing of the ears)
- Sensorineural hearing loss
- Ear plugging sensation
- Autophony
- Deafness
- Other hearing side effects
If you received a Tepezza infusion and experienced hearing loss, it is not too late to hold the drug maker accountable for failing to disclose the risk on the prior Tepezza label.
Find out if you qualify for a Tepezza Lawsuit NO FEES OR EXPENSES UNLESS WE WIN
FDA Adds Hearing Loss Warnings to Tepezza Drug Label
Tepezza (teprotumumab-trbw) was initially approved by the FDA in January 2020, allowing Horizon Therapeutics to market the drug to treat thyroid eye disease symptoms, such as bulging eyes and double vision. These conditions are typically linked to hyperthyroidism and Graves’ disease, leading to inflammation in various parts of the eye.
The Tepezza FDA approval immediately drew criticism, including claims that the clinical trials conducted by Horizon Therapeutics lacked ample population sizes to identify potential side effects for the FDA among diverse groups of patients.
Despite limited pre-market studies, Horizon immediately began to aggressively market and promote the infusions, making Tepezza a blockbuster drug that generated sales in excess of $1 billion a year. However, as users began to experience Tepezza hearing problems, it became clear that the initial label warnings failed to provide critical information for users and the medical community.
July 2023 Tepezza Label Update
On July 20, 2023, the FDA issued an updated version of the prescribing information for Tepezza, which now includes language similar to what lawsuits allege should have been included on the original Tepezza label.
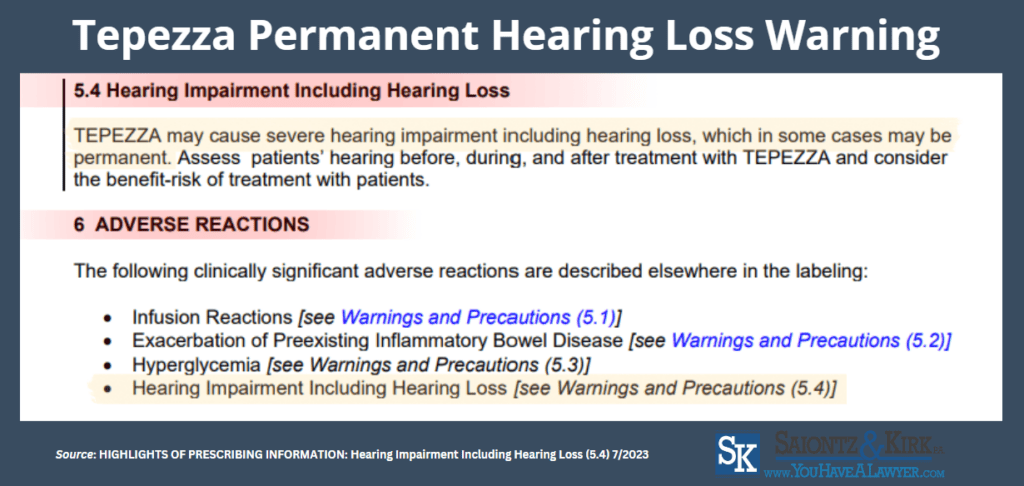
The revised Tepezza advisory now discloses that side effects may cause severe hearing impairment, which is permanent in some cases.
In addition, the language now highlights the importance of healthcare providers assessing patients hearing by conducting a comprehensive evaluation of auditory function prior to initiation of treatment, monitoring hearing throughout the therapy, and reassessing any Tepezza hearing problems post-treatment.
This proactive approach aims to identify and manage any potential hearing related adverse events as early as possible, since many users receive repeated Tepezza infusions.
Why did the FDA update the Tepezza warning label?
Although lawsuits allege that Horizon Therapeutics knew or should have known about the impact of Tepezza on hearing before the drug was ever submitted to the FDA, there has been a steady flow of information since the initial Tepezza FDA label was approved.
The FDA has authority to require changes to a drug’s warning label based on new information that emerges after a drug is introduced to the market. Additional data is continually collected from various sources, including post-marketing surveillance studies, adverse reporting systems and case reports.
If new safety concerns are identified, the FDA can require the drug manufacturer to add new warnings or precautions to the drug’s label, or to change the wording of existing warnings to more accurately reflect the current understanding of the drug’s risks.
Over the past few years, it has become increasingly clear that a Tepezza warning label update was needed.
During the initial clinical trials submitted to the FDA for approval, Horizon data suggested there was only a 10% risk of temporary hearing problems from Tepezza. However, subsequent information has found that real-world Tepezza hearing side effect rates are more than six times what the manufacturer disclosed, with many of the hearing problems persisting long after treatment is stopped.
In March 2021, the results of a significant study were published in the Journal of the Endocrine Society, indicating a notably higher incidence of auditory complications in patients treated with Tepezza than previously estimated by Horizon Pharmaceuticals during their Food and Drug Administration (FDA) approval process.
Specifically, the study demonstrated that 65% of the patients administered with Tepezza experienced some degree of auditory damage or tinnitus. This is a substantially elevated risk, more than six-fold higher than the initial risk projections for hearing loss put forward by Horizon during the FDA regulatory review.
This publication led to subsequent case reports surfacing of individuals who suffered permanent hearing loss after receiving Tepezza, fueling a deeper investigation into the auditory side effects of this medication.
2023 Study Links Tepezza To Increased Hearing Loss Side Effects
In a failed attempt to offset the mounting claims of increased rates of hearing loss side effects from Tepezza, Horizon released a study in May 2023, which analyzed data collected from three separate clinical trials, each involving patients who reported auditory-related adverse events following a 24-week Tepezza treatment regimen. The study was featured in the medical journal, Endocrine Practice.
However, the results of the study revealed that over 16% of Tepezza patients experienced hearing-related complications following their thyroid eye disease treatments. Researchers indicated that 20 patients reported suffering adverse events linked to auditory function while 12 patients reported events that significantly impaired their hearing abilities, including problems such as
- Tinnitus
- Hearing loss
- Autophony
- Eustachian tube dysfunction
- Hypersensitivity or decreased sensitivity to sound (hyperacusis or hypoacusis),
Tepezza Lawsuits Allege Hearing Loss Side Effects Were Ignored
Future users of Tepezza will now have an opportunity to make an informed decision about whether to undergo infusions, and minimize any risk of permanent hearing damage. However, thousands of individuals have received Tepezza since it was initially approved in 2020, and many have now been left with irreversible hearing loss side effects that could have been prevented.
Tepezza hearing loss lawsuits filed against Horizon Therapeutics detail how the drug maker knew or should have known that the mechanism of action for their new treatment could progressively lead to serious hearing loss. Rather than investigating this risk or disclosing information to the FDA, it appears that Horizon placed it’s desire for profits before consumer safety.
Horizon Therapeutics was well aware that Tepezza interferes with the enzyme Insulin-like Growth Factor 1 receptor (IGF1r), which preserve inner ear hair cells that play a crucial role in detecting sound waves and transmitting them to the brain. However, lawsuits allege that the drug maker ignored the risk of permanent hearing problems.
As a result, former users may be entitled to Tepezza hearing loss settlements that provide important financial compensation for damages caused by prior thyroid eye disease treatments, including:
- Pain and Suffering
- Medical Treatment Costs
- Assistance Needed for Hearing Loss
- Lost Wages or Diminished Earning Capacity
- Punitive Damages to Punish Horizon Therapeutics for Failing to Provide Accurate Tepezza Hearing Loss Warnings
Contact Our Tepezza Attorneys
If you or a family member have experienced hearing loss or any other auditory complications after receiving a Tepezza injection, you could be eligible for a settlement or financial compensation. Even if you just have questions about the Tepezza lawsuit, we are here to help. Call our Tepezza lawyers anytime at 1-800-522-0102 or schedule a free consultation through the link below.


3 Comments • Add Your Comments
Julia says:
I wonder why hr. health care service group will not have me relocate to Northampton Manor Nursing home as part time housekeeping and laundry.
Posted on October 12, 2023 at 1:21 pm
Jeffrey says:
Call sientz n kirk. They are the best.
Posted on October 20, 2024 at 1:06 pm
Angelika says:
I was exposed
Posted on November 12, 2024 at 11:07 am