Is There an Impella Heart Pump Lawsuit?
The product liability lawyers at Saiontz & Kirk, P.A. are investigating Impella Heart Pump lawsuits for individuals who have suffered injuries or a wrongful death caused by Abiomed’ s Impella Heart Pump medical devices.
The U.S. Food and Drug Administration (FDA) has issued multiple Impella heart pumps recalls, highlighting risks that the heart pump catheter may cut the wall of the left ventricle in the heart, which has been linked to at least 129 serious injuries and 49 fatalities as of April 2024.
In addition to heart wall tears, officials have also warned that the Impella Heart Pumps may lead to side effects including hypertension, reduced blood flow, blood clots and pump failure resulting in life threatening delays in therapy.
In response to an increasing number of injuries and fatalities tied to the Impella heart pumps, coupled with new information revealing that Abiomed was aware of Impella heart wall tear risks since 2018, lawyers nationwide are currently reviewing potential Impella personal injury lawsuits and wrongful death claims.
Who Qualifies For An Impella Heart Pump Lawsuit? Financial compensation through an Impella Heart Pump lawsuit settlement may be available for individuals who had an Impella blood pump device implanted, and experienced any of the following injuries or life threatening conditions;
- Heart perforation
- Stroke
- Organ failure
- Wrongful death
- Anemia
- Blood clots
- Hypertension
- Vascular damage
- Reduced blood flow
- Hemolysis (Red blood cell destruction)
- Other serious injury
Controversial Approval of Impella Blood Pump Devices
The Abiomed Impella Heart Pump is a left ventricular assist device that is designed to support the heart’s pumping chambers for individuals undergoing major cardiac procedures, including open heart surgery, or those who suffer from severe heart attack, heart failure or cardiogenic shock.
The devices were swiftly brought to market following FDA approval on April 7, 2008, through the controversial 510(k) clearance pathway, which is a regulatory framework that permits the marketing of medical devices by asserting “substantial equivalence” to devices already in use, circumventing the comprehensive testing that a Pre-Market Approval (PMA) requires.
To gain approval, Abiomed successfully argued that the Impella pumps were similar to the existing intra-aortic balloon pumps (IABPs), despite significant operational differences. Unlike the IABPs, which aid the heart through a rhythmically inflating balloon, Impella devices directly relieve the heart by pumping blood from the left ventricle into the aorta, effectively bypassing the heart’s natural pumping action.
However, use of the streamlined approval process for the Impella devices to reach the market has come under scrutiny following a series of FDA recalls and warnings aimed at updating the Impella pumps’ instructions to reduce the risk of injuries and deaths linked to these devices.
Abiomed Impella Heart Pump Recalls
The Impella Heart Pump has been subject to several FDA warnings and recalls in recent years, with the first significant safety statement issued in May 2019, when the FDA reported an elevated mortality rate associated with the Impella RP Heart Pumps.
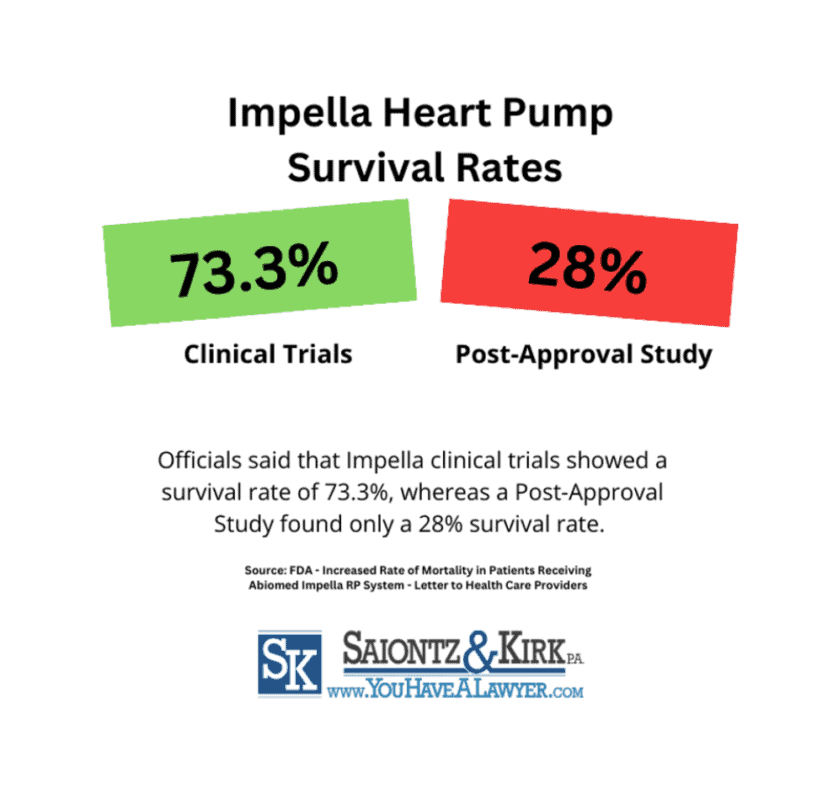
In a warning letter to healthcare providers titled Increased Rate of Mortality in Patients Receiving Abiomed Impella RP System, FDA officials indicated that clinical trials used for the device’s approval showed a survival rate of 73.3%, with 44 out of 60 patients surviving up to 30 days post-device usage.
However, a 2019 post-approval study showed that only 28% of patients using the Impella device survived when transitioning to long-term therapy. A stark difference from the 73.3% survival rate reported by Abiomed. Additionally, this study indicated that only 12 of the 42 patients monitored survived to the 30-day mark post hospital discharge.
After the FDA issued the Impella mortality rate warning, Abiomed contested the findings, arguing that the device was used on a different patient population than in the original clinical trial. Consequently, the FDA retracted this warning, and issued an updated labeling for the Impella RP System in December 2022, warning the devices should not be used in patients with acute right heart failure or decompensation lasting more than 48 hours, or those who present with profound shock, end organ failure, or acute neurologic injury.
March 2024 Impella Recall: Risk of Heart Perforation
The FDA issued a Class I recall for the Impella Left Sided Blood Pump on March 21, 2024, after receiving reports of the Impella catheter piercing the heart’s left ventricular wall.
The recall impacted approximately 66,390 devices, which were previously subject to updated usage guidelines and warnings issued by Abiomed on December 27, 2023, to better communicate the risk of tearing the ventricular wall. However, the FDA later classified this action as a Class I recall, emphasizing the potential for serious injury or fatality.
The devices named in the Impella heart tear recall letter issued by the FDA included the;
- Impella 2.5,
- Impella CP,
- Impella CP with SmartAssist,
- Impella 5.0,
- Impella 5.5 with SmartAssist, and
- Impella LD.
August 2023 Impella Recall: Blood Clot Risk
On August 17, 2023, the FDA announced a Class I Impella blood clot recall due to inadequate instructional labeling of the Impella RP Flex with SmartAssist Catheters for managing patients whose anticoagulation clotting levels are below the recommended threshold.
Officials stated that there have been 12 injuries reported in relation with the recall, and that;
“The use of affected catheters may cause serious adverse health consequences including the risk of blood clots or particle deposits forming or death.”
July 2023 Impella Recall: Pump Failure and Fragment Release
A Class I recall was announced by the FDA on July 27, 2023, for several Impella pump models. The Impella recall was due to the lack of comprehensive instructions for clinicians using the device with transcatheter aortic valve replacement (TAVR) stents.
FDA official stated the existing instructions did not adequately cover the precautions or management tactics necessary for patients with TAVR, which could lead to;
- Total cessation of the pump
- Particles discharged into the bloodstream
- Reduced blood circulation
- Delay therapy
At the time of this recall, 30 complaints had been filed, including reports of 26 injuries and four deaths.
How Do Impella Heart Pumps Cause Death?
Impella heart pumps can cause death through several serious complications, as noted by medical research and FDA recall warnings:
- Heart Wall Perforation: The pump can physically damage and puncture the heart muscle, leading to severe internal bleeding.
- Hemodynamic Instability: The pump can cause significant fluctuations in blood pressure and circulation, which may result in shock and poor blood flow to organs.
- Cardiac Tamponade: Internal bleeding may cause blood to accumulate around the heart, compressing it and significantly reducing its ability to pump blood.
- Organ Failure: Prolonged inadequate blood flow can cause multiple organs to fail, which can be fatal.
Impella Heart Pump Injuries and Side Effects
FDA warnings, recalls, and medical research have highlighted numerous injuries and side effects associated with the use of Impella heart pumps. These complications can range from severe mechanical damage to the heart to systemic issues such as stroke and organ failure.
- Heart Perforation: The Impella catheter is inserted via major blood vessels into the heart and can puncture the left ventricle of the heart, leading to internal bleeding and other complications.
- Stroke: The operation of the pump may dislodge existing blood clots or introduce air bubbles into the bloodstream, which can lead to a stroke if they travel to the brain.
- Organ Failure: In rare cases, reduced blood flow or insufficient blood pressure caused by the device can lead to organ failure, affecting organs such as the kidneys, liver, or heart.
- Hemolysis/Anemia: The Impella device can cause hemolysis, or the mechanical breakdown of red blood cells, which in turn may lead to anemia. This condition can be further compounded by any internal bleeding from heart perforation.
- Blood Clots: The presence of the Impella as a foreign object in the bloodstream can encourage thrombosis or the formation of blood clots. These clots can interfere with the device’s function or cause embolic events if they dislodge.
- Hypertension: The operation of the Impella pump might increase blood pressure due to resistance factors or flow regulation malfunctions, which places additional stress on the cardiovascular system.
- Vascular Damage: Insertion, operation, or removal of the device can occasionally damage vascular walls, leading to complications such as bleeding, thrombosis, or other vascular issues.
- Reduced Blood Flow: The operation of the Impella device might occasionally lead to inadequate blood flow, exacerbating symptoms of heart failure or causing organ damage due to low perfusion.
- Other Serious Injuries: Interactions between the Impella device and a patient’s cardiovascular system can cause various injuries, depending on the individual’s health status and the duration and specifics of the device’s operation.
Impella Heart Pump Side Effects Studies
A growing body of independent research has drawn connections between the use of the Impella device and the emergence of serious and life threatening injuries.
Impella Pump Stroke and Death Risks
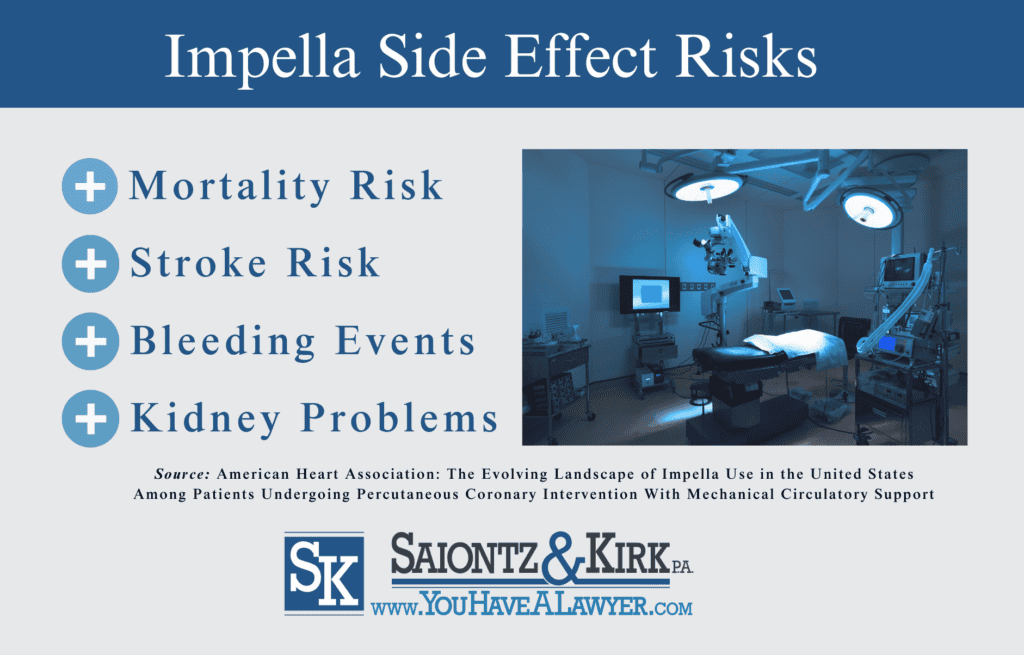
A study published in the American Heart Association in 2019 by Washington University School of Medicine cardiologists found that individuals undergoing percutaneous coronary interventions (PCI) that received an Impella Heart Pump instead of an aortic balloon faced an increased risk of;
- Bleeding events
- Death
- Kidney problems
- Stroke
According to the findings, the Impella Heart Pump device was associated with a 24% higher mortality risk and 34% increased risk of stroke when compared to those who received aortic balloon pumps.
Impella Heart Pump Bleeding Risks
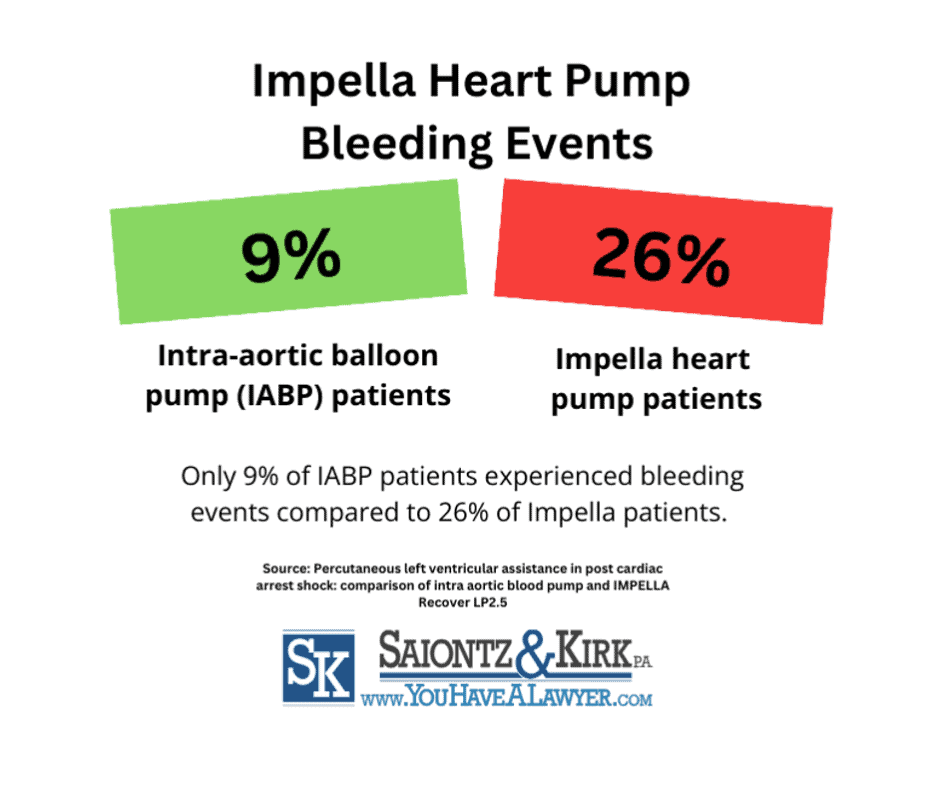
A May 2023 study in the journal Resuscitation found that Impella device users experienced higher rates of serious bleeding compared to those with an intra-aortic balloon pump (IABP). The research analyzed 78 post-cardiac arrest patients, 35 with Impella and 43 with IABP.
Survival rates were 23% for Impella users versus nearly 30% for IABP users. While vascular injuries were similar between groups, 26% of Impella patients suffered serious bleeding, compared to just 9% of IABP patients.
Impella Lawsuit FAQs
What is the Impella Heart Pump and Connect System?
The Impella left sided blood pumps consist of a pump catheter, which is a thin, flexible tube that houses a microaxial blood pump. This catheter is inserted through the femoral artery and threaded into the heart, specifically into the left ventricle.
The pump at the end of the catheter helps by drawing blood from the left ventricle and expelling it into the ascending aorta, effectively unloading the heart and increasing blood flow to the rest of the body.
The device is also equipped with an Impella Connect System. This feature is a cloud-based remote monitoring platform that allows healthcare professionals to observe and manage the device’s performance in real-time. It provides data and visualizations for medical professionals to make informed decisions about patient care.
Which Impella Heart Pump models have been recalled?
- Impella 2.5
- Impella CP
- Impella CP with SmartAssist
- Impella 5.0
- Impella 5.5 with SmartAssist
- Impella LD
- Impella RP Flex with SmartAssist
What do I do after suffering an Impella Heart Pump injury?
If you or someone close to you has been harmed by the Impella Heart Pump, it’s important to act quickly to secure your health and uphold your legal rights.
Here are first steps to take if you or someone you love has been injured by an Impella Heart Pump:
- Seek Immediate Medical Attention: Get a medical evaluation to assess and record any injuries caused by the Impella Heart Pump.
- Report the Incident to the FDA: Notify the FDA’s MedWatch program about the incident. This can be done online, or by mail or fax, to help monitor and address adverse effects related to medical devices.
- Gather and Preserve Evidence: Save all medical documents, device details, and any communication regarding your use of the Impella Heart Pump.
- Keep a Detailed Record of Your Experience: Document all symptoms, medical visits, and the overall impact of the injury on your life.
- Contact a Lawyer Specializing in Medical Device Litigation: Consult with a lawyer who specializes in medical device cases to explore your legal options and consider pursuing a claim against the device manufacturer.
What damages can I receive through an Impella lawsuit settlement?
- Medical Expenses: Compensation for all health-related costs you have incurred due to the complications from the Impella device. This includes hospital bills, costs of diagnostic tests, medication, and any other medical treatments needed to address the complications caused by the device.
- Future Medical Treatment: Often, injuries caused by medical devices like the Impella heart pump can require prolonged or even lifelong medical treatment. You can claim compensation for estimated future medical expenses to ensure ongoing care and rehabilitation.
- Lost Wages: If the complications from the Impella device have resulted in time away from work, you may receive compensation for lost wages. This includes payment for the time you were unable to work and, if applicable, loss of future earning capacity.
- Pain and Suffering: This covers the physical pain and emotional distress suffered due to the injuries. Pain and suffering are calculated based on the severity of the pain and the prognosis of recovery.
- Wrongful Death: If complications from the Impella device result in death, family members or the estate of the deceased may pursue a wrongful death claim. This can include compensation for funeral and burial expenses, loss of future income, loss of companionship, and the emotional pain suffered by surviving family members.
Are There Any Costs to Hire a Impella Lawyer?
There are absolutely no out-of-pocket costs to review your case or hire our attorneys. Potential claims are evaluated for individuals throughout the United States, and all cases are handled on a contingency fee basis.
Through the use of contingency attorney fees, individuals have access to the experience and resources of our national law firm for their Impella lawsuit — regardless of their individual financial resources.
You pay nothing up front to hire our lawyers, and we only receive an attorney fee or expenses out of the money that is obtained from the manufacturers. Our law firm receives nothing unless we win your case!
What are the steps in an Impella case evaluation?
Complete Our Case Evaluation Request Form. Providing contact information and some information about your Impella injury case.
Get Contacted by Saiontz & Kirk, P.A. You will be contacted by our law firm to help determine if financial compensation may be available for you and your family.
You Decide If You Want to Move Forward. If our lawyers determine that we can help with your case then you decide whether to move forward and hire us to pursue compensation.
