Is There a Bard PowerPort Lawsuit?
Yes. Bard PowerPort fracture and migration lawsuits are being filed against the manufacturers of Bard PowerPort devices over design problems that can cause the catheter’s ChronoFlex material to fissure, fracture or crack.
The design defects of the Bard PowerPort catheters have been linked to a growing number of serious injuries and fracturing events, in which small fragments of the catheter broke free in the patient’s vascular system, leading to fatalities, infections, blood clots and emergency surgical procedures to retrieve the fragments.
Similar to other port catheter lawsuits filed in recent years over defective designs, Bard PowerPort lawsuits claim that life threatening injuries could have been avoided if a safer alternative design had been used, or if the manufacturer had warned patients and doctors by issuing a Bard PowerPort recall.
Who is the Bard PowerPort lawsuit against?
The Bard PowerPort lawsuit is against Becton Dickinson and Company, and its subsidiaries, C.R. Bard. Inc. and Bard Access Systems Inc.
Who Qualifies For A Bard PowerPort Lawsuit? Financial compensation through a Bard PowerPort lawsuit settlement may be available for individuals who had a Bard PowerPort implanted, and experienced any of the following injuries or conditions;
- Perforations of tissues, vessels and organs
- Infections (sepsis or septic shock)
- Deep Vein Thrombosis (DVT)
- Hemorrhaging or Bleeding Injuries
- Fluid buildup on the heart
- Irregular Heartbeat
- Severe and persistent pain
- Wrongful death
- Other injuries caused by fractured PowerPort catheter
What models are included in the Bard Implantable Port Lawsuit?
In addition to lawsuits over Bard PowerPort implantable port, claims are also being investigated for individuals who suffered injuries caused by any of the following Bard access systems;
- Bard PowerPort
- Bard Powerflow™
- Bard SlimPort
- Bard X‑Port™
- Bard MRI Ports
- Bard Groshong Catheter
- Bard Titanium Port
- Bard Vaccess CT
2024 Bard PowerPort Lawsuit Updates
March 14, 2024 Update: Judge Campbell issued a case management order (PDF) on March 11, 2024, denying the manufacturer’s request to delay Bard PowerPort bellwether trials. In the order, Judge Campbell affirmed that the current discovery expectations in the litigation will remain intact and that the bellwether process will continue as planned. After the discovery phase, the parties aim to reach a consensus on the final six bellwether claims by March 10, 2025, with the anticipation that the initial trials could begin towards the end of 2025 or the beginning of 2026.
February 2024 Update: On February 5, 2024, the U.S. JPML issued a transfer order (PDF) that broadened the scope of the ongoing multidistrict litigation (MDL) concerning Bard PowerPort catheter lawsuits. This expansion encompasses lawsuits not only regarding issues with the PowerPort catheters but also those concerning infections resulting from defects in the Bard PowerPort’s port reservoir. Previously, the MDL was confined to claims solely concerning the catheters. As a result of this expansion, all federal lawsuits involving both catheter and port reservoir problems, including those alleging infections from PowerPort reservoir defects, will now fall under the jurisdiction of the Bard Implanted Port Catheter Products Liability multidistrict litigation.
January 2024 Update: According to a case management order (PDF) issued on January 9, 2024, the U.S. JPML is scheduled to hear oral arguments this month on whether to include a growing number of Bard PowerPort reservoir failure lawsuits to the ongoing multidistrict litigation. While the Bard PowerPort lawsuits have focused mainly on the alleged catheter design defects, several complaints have been filed in recent weeks stating the Bard PowerPort reservoir is made of a nondurable plastic material that can become weak and prone to fracturing, or harboring bacteria that can lead to severe infections.
August 2023 Update: With at least 50 Bard PowerPort lawsuits filed across 28 different federal districts, the U.S. JPML issued a transfer order on August 8, 2023, ordering all Bard PowerPort fracture and migration claims to be centralized for coordinated pretrial proceedings in the U.S. District for the District of Arizona before Judge David G. Campbell.
May 2023 Update: With a rapidly growing number of Bard PowerPort injury or wrongful death lawsuits currently pending in various U.S. District Court nationwide, a petition has been filed with the U.S. Judicial Panel on Multidistrict Litigation asking the Panel to consolidate the claims into one centralized multidistrict litigation, where the cases will be managed similar to a Bard PowerPort class action lawsuit during discovery and pretrial proceedings.
April 2023 Update: Several Bard PowerPort lawsuits have been filed in recent months claiming the chemical makeup of the ChronoFlex catheters used in conjunction with PowerPort™, Powerflow™, SlimPort™ and X-Port™ implantable access systems are prone to cracking, fracturing and fissuring. Many of the lawsuits claim the fracturing of the catheter port caused individuals to develop serious injuries and the need for emergency surgical intervention.
Bard PowerPort Lawsuit Info On This Page
What is a Bard PowerPort Device?
How do I know if I have a Bard PowerPort?
What is Wrong with Bard PowerPort Devices?
Bard PowerPort Complications and Injuries
Is There a Bard PowerPort Class Action Lawsuit?
Bard PowerPort Settlement Information
Steps in Hiring a Bard PowerPort Lawyer
What is a Bard PowerPort Device?
The Bard PowerPort device, also known as a TIVAD, port-a-catheter, or “Port a Cath,” is a medical device designed for subcutaneous vascular access. It facilitates repeated access to the peripheral vein for the delivery of intravenous therapies, such as medications, fluids, and parenteral nutrition solutions.
The port is typically implanted under the skin on either the chest or arm, where the catheter can access one of the large central veins that take blood to your heart.
The FDA approved the Bard PowerPort TIVAD in 2000 for use in treatments requiring repeated IV access, such as chemotherapy, long-term antibiotic therapy, total parenteral nutrition, and pain management.
What is the Bard power port made of?
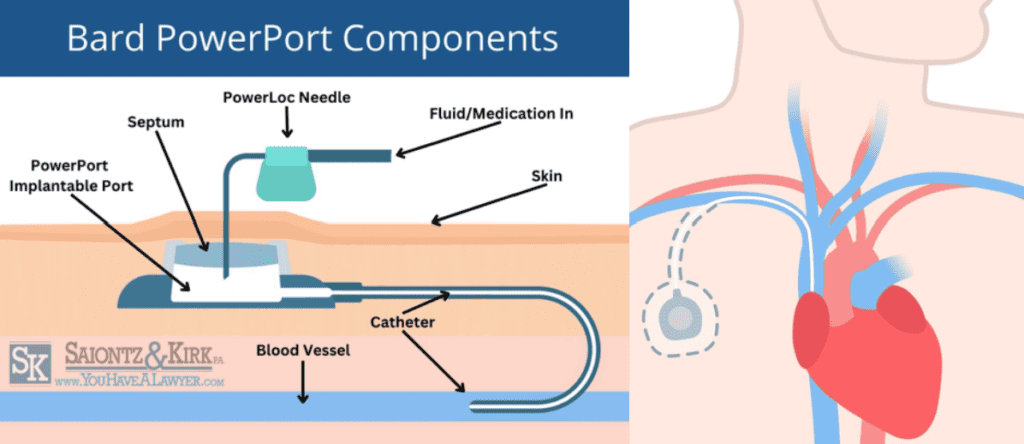
Bard TIVAD’s consist of four primary components:
PowerPort Body or Reservoir: This is the main part of the PowerPort and it is made from a biocompatible material such as titanium or plastic. The injection port reservoir can be accessed through the skin with special needles for the delivery of medication or drawing blood samples. It is typically round or oval, and about the size of a quarter.
Septum: This is a self-sealing silicone component with a raised center located on the top of the port body. Needles can be inserted through the septum into the implanted port for medication delivery or sampling blood products, and the septum self-seals once the needle is removed.
PowerLoc Needle: The PowerLoc needle allows medications to be injected directly into the port’s reservoir, from where they can travel through the attached catheter and into the patient’s bloodstream
Catheter Tube: The polyurethane catheter is a flexible tube made from a ChronoFlex material. The catheter connects the port body to the vein, providing intravenous fluids direct access from the injection port to the blood vessel. The other end of the catheter is inserted into a large central vein, typically the superior vena cava, for direct access to the bloodstream.
What is a Bard PowerPort for chemotherapy?
Bard PowerPorts are power injectable ports commonly implanted in cancer patients receiving chemotherapy treatments to administer dyes and contrast agents at a high rate of flow.
The high injection flow rates of the contrast agents help improve the clarity of the images from CT scans, MRI, and PET scans, which allows a doctor to differentiate between normal and abnormal tissues, clearly outline the boundaries of a tumor, and assess how well the treatment is working.
The Bard PowerPorts are often implanted in individuals who will require chemotherapy over a longer period of time. Bard PowerPorts are frequently used during chemotherapy for the following cancers;
- Breast Cancer
- Lung Cancer
- Stomach Cancer
- Pancreatic Cancer
- Liver Cancer
- Esophageal Cancer
- Gallbladder Cancer
- Colon Cancer
- Leukemia
- Non-Hodgkin lymphoma
- Hodgkin lymphoma
- Multiple myeloma
- Myelodysplastic Syndromes (MDS)
How do I know if I have a Bard PowerPort?
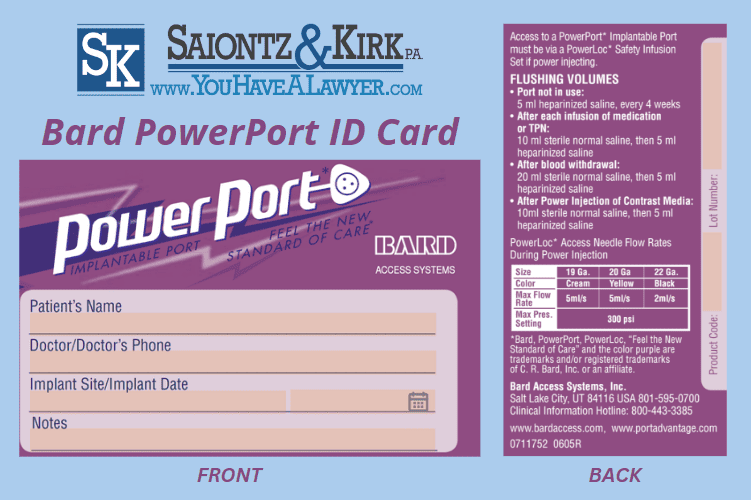
Patients are issued a Bard PowerPort ID Card by their doctor or nurse that contains critical information about the device, including the manufacturer’s name, device model, serial number, and the date of implantation. The PowerPort ID is issued to allow doctors to identify the type of device a patient has since all implantable ports are not rated for the same rate of pressure.
Recipients may have also been issued a rubber PowerPort bracelet which has different colors indicating the type of implantable port the patient received.
Bard PowerPort ID Card
What is wrong with Bard PowerPort devices?
Lawsuits allege that the problems with Bard PowerPort catheters stem from the use of high concentrations of barium sulfate, which weakens the strength of the catheter, allowing microfractures, deterioration, fissuring, and cracking.
These catheter fractures cause small fragments to travel throughout the bloodstream resulting in vascular damage and other serious injuries.
Becton Dickinson and its subsidiaries designed many of the PowerPort devices to use catheters made of a material called “ChronoFlex”, which is a blend of polyurethane and barium sulfate.
Improper mixing and excessive use of barium sulfate during the manufacturing process may cause pockets of the chemical compound and entrapped air to form through the PowerPort’s catheter body and inner and outer surfaces, according to lawsuits now being pursued. These pockets in the Bard PowerPort catheter may deteriorate over time, leaving irregular surfaces for microbes to collect and cause infections, as well as fractures and other complications.
Studies Linking PowerPort Designs to Fractures and Failures
Numerous studies have suggested that the use of barium sulfate in PowerPort and other Totally Implanted Venous Access Devices (TIVADs) can lead to a heightened risk of fractures, infections and mechanical failures.
A study published in the Journal of Biomedical Materials Research noted that barium sulfate particles do not fully integrate into polymer catheters, which can increase the risk of surface irregularities and increased thrombogenicity, which refers to the ability of a material to generate blood clotting and thrombus.
Just several years later, a study was published in the Journal of Mechanical Behavior of Biomedical Materials finding the use of barium sulfate in TIVAD catheters can increase the risk of mechanical failures and fractures. The researchers found that the loss of barium sulfate particles under stress creates notches, acting as predetermined breaking points, which can lead to mechanical failure.
Safer Design Alternatives Were Available
In a study published in Nephrology Dialysis Transplantation in 2010, researchers found that the release of barium sulfate contributes to catheter surface defects that could increase the risk of fracturing and bacterial proliferation. Researchers stated that a coating of polyurethane could prevent these issues causing injuries to patients.
Despite the ability to reduce or prevent fractures by changing the design to encapsulate the radiopaque compound or use a different polymer formulation, lawsuit claim the manufacturer continued to actively and aggressively market the PowerPort as safe.
Bard PowerPort Complications and Injuries
As a result of the defective and unreasonably dangerous design, a growing number of Bard PowerPort catheter infections and fractures have been reported in recent years.
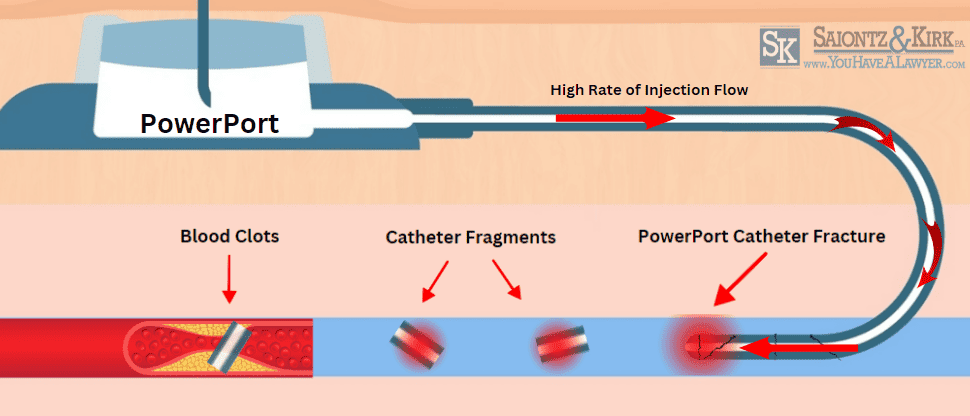
Bard PowerPort Catheter Fractures
The ports intended high rate of injection flow combined with the fragile structure of the PowerPort may cause catheter fractures that allow tiny fragments of plastic tubing to break off and enter a patient’s bloodstream. This could lead to serious and potentially fatal health issues, including;
- Blood clots
- Irregular heart beat (tachycardia)
- Cardiac punctures
- Pulmonary embolism
- Tearing of blood vessels
Bard PowerPort Catheter Migration
A Bard catheter migration occurs when the flexible tube, which is used to access the body cavities and vascular system, moves from its intended position to another location in the body. This can cause severe health problems such as:
- Obstruction of blood flow
- Infection
- Organ perforation
- Organ damage
- Catheter failure
Bard PowerPort Infections
Users may be at risk of a Bard catheter infection due to bacteria building up in the small fissures and cracks caused by the breakdown of the barium sulfate compound. These infections can have life-threatening consequences for cancer patients undergoing chemotherapy treatments, as well as other individuals who already have weakened immune systems.
Some of the most common infections that could arise from a fractured or migrated PowerPort include;
- Klebsiella
- Staphylococci
- Candida
Symptoms of infection from a Bard PowerPort may include:
- Fever & Chills
- Inflammation and Swelling
- Drainage or pus
- Changes in urine color or odor
- Confusion
Bard PowerPort Complications Were Known for Years
According to allegations being pursued in Bard PowerPort lawsuits, Becton Dickinson and its subsidiaries have known about the high rate of the catheters fracturing, migrating, and causing infections and other complications since the early 2000’s.
It appears that the manufacturer placed its desire for profits before consumer safety, by concealing thousands of reports of PowerPort failures and injuries from doctors and patients, by taking advantage of the FDA’s controversial Alternative Summary Reporting (ASR) program.
The FDA’s ASR program allowed the manufacturers to hide the high rate of device failures and injuries from the public through exemptions, variances, or alternatives under 21 CFR 803.19.
However, unlike the public Manufacturer and User Facility Device Experience (MAUDE) database, manufacturer reports of device failures submitted through ASR were not accessible publicly, including healthcare providers, until 2019.
Is There a Bard PowerPort Class Action Lawsuit?
To date, there is not currently a Bard PowerPort class action lawsuit that seeks damages for all individuals who received the port catheter. However, a Bard PowerPort multidistrict litigation was established in August 2023, in an effort to efficiently manage the rising number of claims each raising similar allegations against the manufacturer.
Due to the surging number of Bard PowerPort fracture and migration lawsuits in various federal courts, the U.S. Judicial Panel on Multidistrict Litigation (JPML) issued a transfer directive (PDF) on August 8, 2023. This directive was a response to a request to consolidate the claims, directing all PowerPort lawsuits to be overseen by one judge during pretrial stages.
While each Bard PowerPort lawsuit will remain its own individual case, the claims will be consolidated in the U.S. District for the District of Arizona before Judge David G. Campbell. In steering the Bard Port lawsuits MDL, it’s anticipated that Judge Campbell will initiate a bellwether process, which will involve selecting a subset of representative claims for case-specific discovery and setting early trial dates.
Allegations Raised in Bard PowerPort Lawsuits
Individuals throughout the United States are currently pursuing product liability lawsuits against Becton Dickinson and its subsidiaries raising similar allegations, including;
- Failure to adequately design and test the durability and strength of the material used to make Bard PowerPort catheters;
- Falsely advertised and misrepresented the safety of the port devices;
- Failure to disclose reports of the PowerPort colonizing bacteria, fracturing and malfunctioning;
- Failure to provide adequate warnings to healthcare providers about the unreasonably dangerous and defective design of the implanted port device;
- Failure to issue proper warnings or issue a PowerPort recall.
Bard PowerPort Settlement Information
As a direct result of the manufacturer’s apparent faulty design of the Bard PowerPort catheter, and the desire to put profit before the health and safety of patients, PowerPort lawsuit settlements may become available for individuals left with debilitating injuries.
Although the manufacturer has not agreed to any global settlement for Bard PowerPort lawsuits, our product liability lawyers believe this will change as the discovery process moves forward and individual cases approach trial.
Saiontz & Kirk, P.A. is continuing to review new Bard PowerPort lawsuits for individuals who received one of the implanted port devices who experienced complications that resulted in serious injuries or death.
How Much Is A Bard PowerPort Settlement Worth?
While no global Bard PowerPort lawsuit settlements have been reached yet, our lawyers expect that the future value of an individual PowerPort settlement will depend on several factors. For example, individuals who experienced complications resulting in permanent injuries may be entitled to higher settlement payouts than those who fully recover.
In determining the amount of any individual PowerPort lawsuit payout, the following are some of the common factors that will be taken into consideration when negotiating or evaluating any offers:
- The specific injuries caused by the PowerPort;
- The amount of any past or future medical expenses;
- The amount of any lost wages or loss of earning capacity;
- The level of impact the PowerPort has caused on the individual’s overall physical and mental health or well-being;
- The past and future amount of pain and mental anguish suffered by the individual;
Are There Any Costs to Hire a Bard PowerPort Lawyer
There are absolutely no out-of-pocket costs to review your case or hire our attorneys. Potential claims are evaluated for individuals throughout the United States, and all cases are handled on a contingency fee basis.
Through the use of contingency attorney fees, individuals have access to the experience and resources of our national law firm for their Bard PowerPort lawsuit settlement — regardless of their individual financial resources.
You pay nothing up front to hire our lawyers, and we only receive an attorney fee or expenses out of the money that is obtained from the manufacturers. Our law firm receives nothing unless we win your case!
What are the steps in a PowerPort case evaluation?
Complete Our Case Evaluation Request Form. Providing contact information and some information about your PowerPort injury case.
Get Contacted by Saiontz & Kirk, P.A. You will be contacted by our law firm to help determine if financial compensation may be available for you and your family.
You Decide If You Want to Move Forward. If our lawyers determine that we can help with your case then you decide whether to move forward and hire us to pursue compensation.
