2023 Eye Drops Recall Lawsuit
Lawyers are investigating Artificial Tears eye drop lawsuits following a nationwide outbreak of Pseudomonas aeruginosa infections in 2023, which have been linked to certain Delsam Pharma and EzriCare eye drops products.
Consumers are being warned that the contaminated eye drops have caused dozens of users to be hospitalized with bacterial infections, with some resulting in permanent vision loss or fatal bloodstream infections. Following an investigation launched in January 2023, the CDC confirmed the presence of antibiotic-resistant bacteria in opened bottles of hospitalized Artificial Tears users.
The findings prompted the U.S. Food and Drug Administration (FDA) to issue an Artificial Tears recall on February 2, 2023, and an Artificial Eye Ointment recall on February 24, 2023.
As a result of the manufacturers apparent failure to adequately test and ensure the ointment and eye drops were free of harmful bacteria, lawyers are now pursuing Artificial Tears recall lawsuits and Artificial Eye Ointment recall lawsuits for individuals who have suffered injuries.
Financial compensation through an Artificial Tears eye drops settlement may be available to consumers who used any of the following products after June 1, 2022;
- EzriCare Artificial Tears,
- Delsam Pharma eye drops, or
- Delsam Pharma Artificial Eye Ointment
And were diagnosed with;
- Eye Infection
- Partial Vision Loss
- Permanent Vision Loss
- Eye Injuries
- Bloodstream Infection
- Other injuries caused by the eye drops
Latest Eye Drops Recall Lawsuit Updates: 2023
February 24, 2023 Update: The FDA announced a Delsam Pharma Artificial Eye Ointment recall, warning the products may contain the same pseudomonas aeruginosa bacteria that was found in Artificial Tears eye drops. The eye ointment recall further warns some of the product packaging may leak, creating additional contamination risks.
February 6, 2023 Update: Following the recall announcement, lawyers are now pursuing eye drop lawsuits against the manufacturers for individuals who have suffered infections resulting in vision loss or other injuries.
February 2, 2023 Update: The FDA announced an Artificial Tears recall for EzriCare and Delsam Pharma’s brand name eye drops, warning the products may contain an antibiotic resistant strain of pseudomonas aeruginosa bacteria that may lead to serious and life threatening side effects.
January 20, 2023 Update: The CDC released an EzriCare Artificial Tears infection warning, warning consumers to immediately stop using the lubricating eye drops as the agency continues to investigate several clusters of pseudomonas aeruginosa infections across multiple states.
EzriCare Eye Drops Recall Information
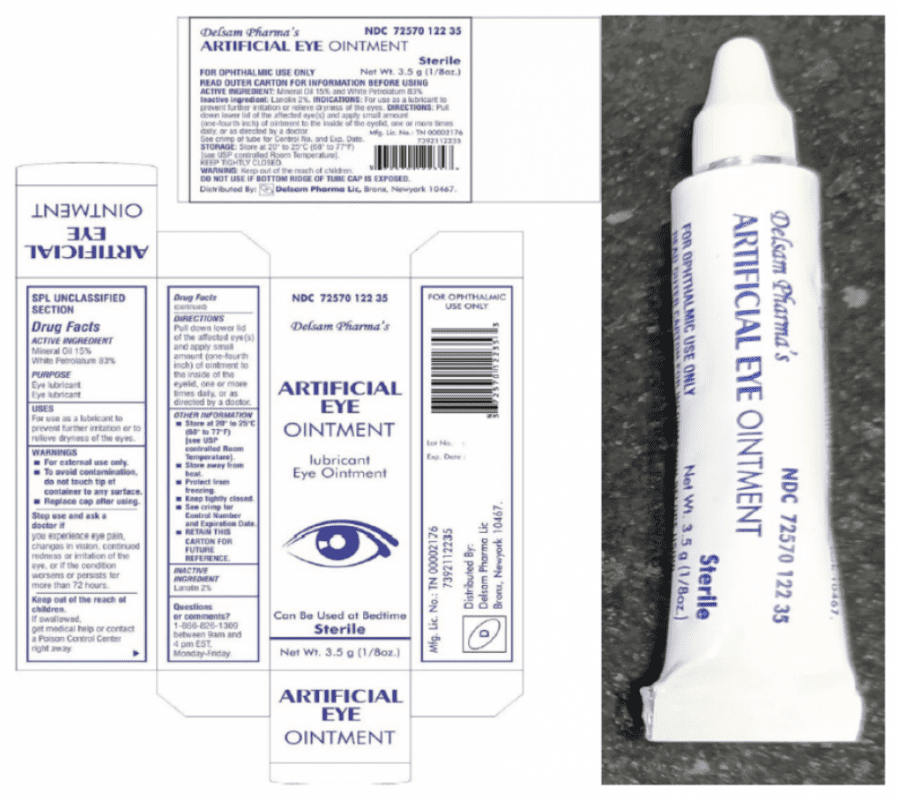
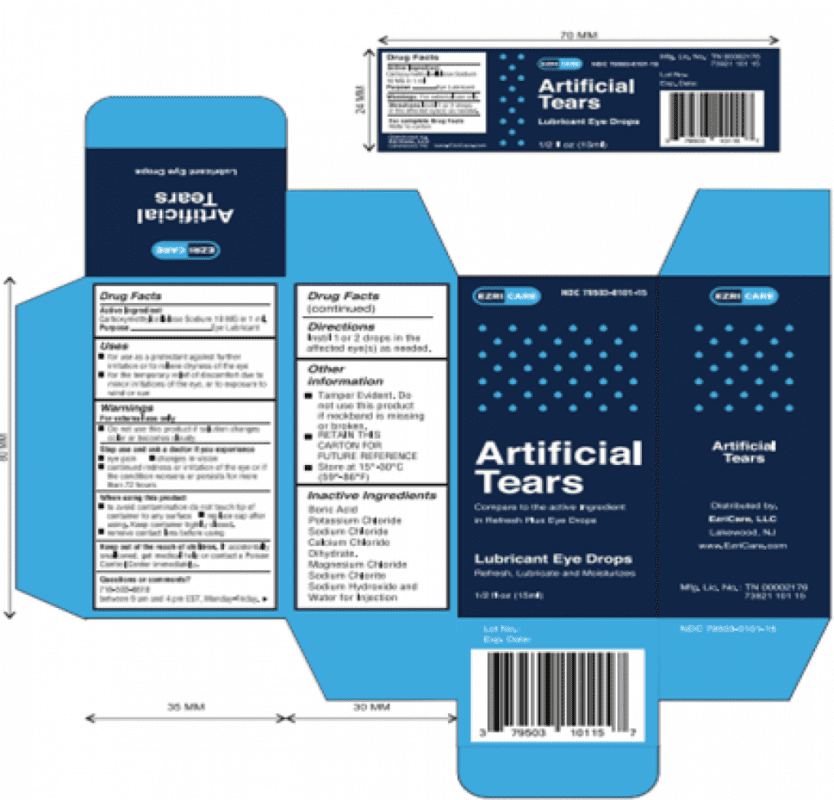
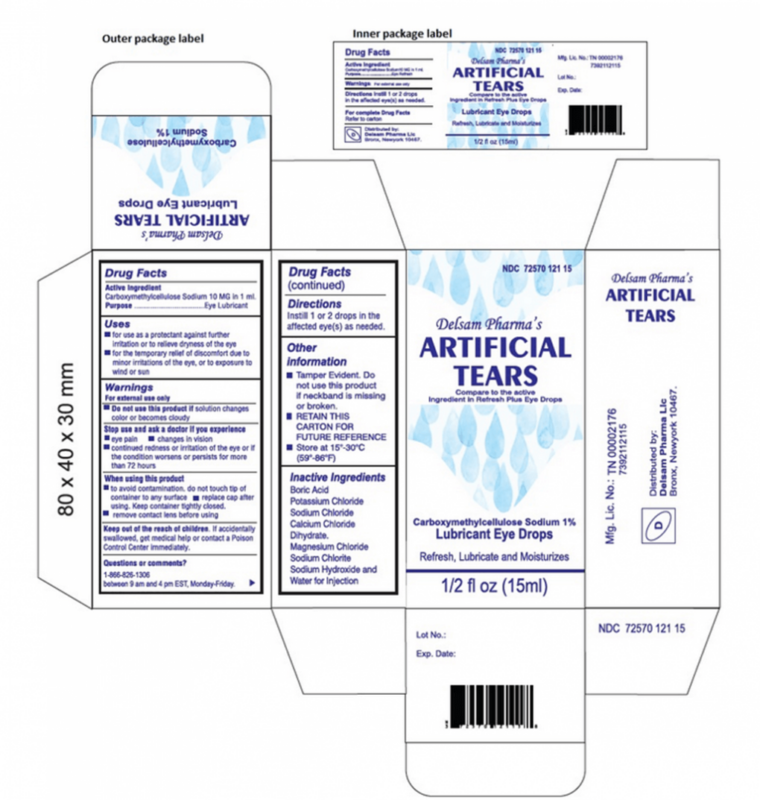
The FDA issued the Artificial Tears eye drops recall on February 2, 2023, instructing customers to stop using EzriCare and Delsam Pharma Artificial Tears Lubricant Eye Drops that were sold in ½ fluid ounce (15 ml) bottles. Just three weeks later, the FDA announced a Delsam Pharma Artificial Eye Ointment recall on February 24, 2023, warning the products may contain the same bacterial contamination found in Artificial Tears.
The recalls include the follow Artificial Tears and Eye Ointment products;
- EzriCare Artificial Tears (blue box): NDC 79503-0101-15 with UPC 3 79503 10115 7
- Delsam Pharma Artificial Tears (white box): NDC 72570-121-15 with UPC 72570-0121-15
- Delsam Pharma Artificial Eye Ointment (white bottle): NDC 72570-122-35, with UPC 3 72570 012235 3
Artificial Tears Eye Drop Infection Risks
Federal health officials have identified a wide variety of injuries caused by the recalled eye drops, warning that pseudomonas aeruginosa infections may cause;
- Eye infections
- Permanent vision loss
- Respiratory infections
- Urinary tract infections
- Fatal bloodstream infection
2023 Eye Infections From Artificial Tears Eye Drops
The recalled Artificial Tears have been linked to an increased risk of eye infections that may result in deterioration of the eyeball, causing total blindness. In early 2023, the CDC stated the antibiotic resistant strain of bacteria at hand has limited treatment options and has already caused a portion of hospitalized consumers to suffer permanent vision loss.
Health officials warned that eye drop users should be aware of the following potential symptoms of eye infections after using EzriCare Artificial Tears;
- Blurred vision
- Swollen eyes
- Eye pain
- Redness of the eyes
- Ocular discharge
- Watery eyes
- Dry eyes
- Sensitivity to light
- Itching
Bacteria Found In EzriCare Eye Drops
Officials have confirmed the presence of Carbapenem-Resistant Pseudomonas Aeruginosa (CRPA) in the recalled Artificial Tears products, which is a superbug that is typically found in healthcare settings and acute care facilities, such as hospitals.
This bacterium is resilient to antibiotics, making it difficult to treat and potentially deadly for vulnerable individuals. CRPA frequently develops resistance to carbapenems, which are powerful antibiotics used to treat serious, life-threatening bacterial infections.
How Do Artificial Tears Cause Vision Loss?
Artificial Tears containing Pseudomonas aeruginosa can cause a rare eye condition known as endophthalmitis that can lead to vision problems if left untreated. In severe cases, the condition causes inflammation and deterioration of the tissue throughout the eye which can cause irreversible blindness.
Artificial Tears Eye Drop Lawyers
The personal injury lawyers at Saiontz & Kirk, P.A. provide free consultations and case evaluations to help individuals review the legal options that are available to them. After contacting our office toll-free at 1-800-522-0102 or requesting a free case review online, the facts and circumstances surrounding your potential eye drops case will be reviewed and evaluated by our lawyers.
If it is determined that you or a loved one may be eligible for financial compensation or a settlement, it is your decision whether to hire our law firm. All eye drop lawsuits are handled by our experienced product liability layers under a contingency fee agreement, which means that there are never any fees or expenses paid unless we are successful in obtaining a settlement or other recovery in your case.