Is There a Mounjaro Side Effects Lawsuit?
Yes, people across the U.S. are investigating potential lawsuits against Mounjaro manufacturers, after learning that this medication may cause stomach paralysis and other serious gastrointestinal complications.
Thousands of adverse health events have been reported to the U.S. Food and Drug Administration (FDA) by individuals who received Mounjaro injections, leading many individuals to question whether Eli Lilly and Company adequately researched the extent and severity of Mounjaro.
Allegations raised in Mounjaro lawsuits claim the manufacturer did not sufficiently investigate their new drug and failed to alert doctors and patients about potential gastroparesis side effects.
Is there a Mounjaro Class Action lawsuit?
The U.S. Judicial Panel on Multidistrict Litigation issued a transfer order on February 2, 2024, instructing all GLP-1 stomach paralysis lawsuits involving Mounjaro, Ozempic, Wegovy and other similar GLP-1 drugs to be centralized in the U.S. District Court for the Eastern District of Pennsylvania before U.S. District Judge Gene E. K. Pratter for coordinated pretrial proceedings.
If you or a loved one has suffered gastrointestinal injuries after receiving Mounjaro, you may be eligible for compensation through a lawsuit claim.

Who Qualifies for a Mounjaro Lawsuit?
Individuals may qualify for a Mounjaro lawsuit if they received tirzepatide injections and suffered any of the following complications:
- Gastroparesis (Stomach Paralysis)
- Excessive vomiting
- Severe diarrhea
- Severe abdominal pain
- Gastrointestinal burning
- Gastric obstruction
- Gastroesophageal Reflux Disease (GERD)
- Other Gastrointestinal injuries
Our lawyers are also pursuing Ozempic lawsuits and Wegovy lawsuits for individuals who developed similar gastrointestinal injuries after taking medications containing the ingredient semaglutide. Potential gastroparesis lawsuits are also being investigated for users of the tablet form of semaglutide, which was marketed as Rybelsus.
To help determine whether you or a loved one may be eligible to receive a Mounjaro settlement or lawsuit payout, request a no-obligation case evaluation today.
2024 Mounjaro Lawsuit Update
Following recent studies and case reports linking GLP-1 receptor agonist drugs such as Mounjaro and Ozempic to gastrointestinal problems, attorneys are reviewing Mounjaro side effects lawsuits.
- February 2024 Update: The U.S. JPML dismissed Eli Lilly’s opposition and issued a transfer order (PDF) on February 2, 2024, calling for all Mounjaro lawsuits and other GLP-1 lawsuits filed over stomach paralysis side effects to be centralized before U.S. District Judge Gene E.K. Pratter in the U.S. District Court for the Eastern District of Pennsylvania for coordinated pretrial proceedings. As part of the Glucagon-Like Peptide-1 Receptor Agonist Products Liability Litigation, it is anticipated that a group of representative claims will be selected for a series of early bellwether trials to determine how juries will respond to similar evidence repeated throughout the claims.
- January 2024 Update: Eli Lilly has filed a response opposing the motion to consolidate Mounjaro stomach paralysis lawsuits into a multidistrict litigation with other GLP-1 drugs, claiming that the majority of lawsuits involve Novo Nordisk’s Ozempic and Wegovy drugs, and that most claims filed to date involve plaintiffs who used the drugs for weight loss.
- December 2023 Update: A motion to transfer (PDF) has been filed with the U.S. Judicial Panel on Multidistrict Litigation requesting that all Ozempic, Saxenda, Trulicity and Mounjaro lawsuits filed over gastroparesis side effects be consolidated before a single judge in the Western District of Louisiana.
- October 2023 Update: The findings of a study published in the Journal of the American Medical Association (JAMA) on October 5, 2023, revealed that injectable GLP-1 agonist drugs such as Ozempic, Wegovy, and Mounjaro carry a threefold increased risk of stomach paralysis (gastroparesis) when compared to those using alternative weight loss medications.
- August 2023 Update: A Louisiana woman filed the first Mounjaro lawsuit on August 2, 2023, raising allegations that Eli Lilly failed to warn the medical community about the risk of severe stomach problems. The lawsuit claims that as a result of the failure to warn, she has developed gastroparesis after using both Mounjaro and Ozempic diabetes medications.
What is Mounjaro?
Tirzepatide, marketed under the brand name Mounjaro, is a medication that was developed by Eli Lilly and Company and approved by the U.S. Food and Drug Administration in May 2022, for the management of type 2 diabetes.
Mounjaro falls under a class of diabetes medicines known as glucagon-like peptide-1 (GLP-1) receptor agonists, which work to regulate blood sugar levels in those with type 2 diabetes by mimicking the action of the GLP-1 hormone to stimulate insulin after consuming food.
What does Mounjaro do to the body?
Mounjaro interacts with a specific protein in the body called the GLP-1 receptor. When this protein is activated by Mounjaro, it helps the body produce more insulin, which lowers blood sugar. At the same time, it decreases the release of glucagon, another hormone that can raise blood sugar when there’s too much of it.
Mounjaro also causes delayed gastric emptying by slowing down the movement of food from the stomach to the intestines to make individuals feel fuller for longer. These combined effects have been approved by the FDA to control blood sugar levels among those with type 2 diabetes. However, the delay in gastric emptying from Mounjaro may also cause users to be hospitalized with stomach paralysis or other gastrointestinal injuries.
Is Mounjaro the same as Ozempic?
Mounjaro is similar to Ozempic in the sense that both drugs promote insulin secretion by mimicking the action of the hormone GLP-1, which is secreted by the L-cells of the intestine in response to food intake. However, Mounjaro also targets the glucose-dependent insulinotropic polypeptide (GIP) hormone, that is secreted by the K-cells found in the proximal small intestine.
Is Mounjaro approved for weight loss?
No. Mounjaro is currently only approved as a treatment for type 2 diabetes. However, the drug has become widely used for off label purposes as an obesity medicine for weight loss similar to Ozempic and other semaglutide drugs offered by Novo Nordisk since 2017.
Problems With Mounjaro
Lawsuits claim that Eli Lilly and Company recognized the billions of dollars being made by Novo Nordisk from the off-label weight loss use of Ozempic and sought to capitalize on the success by releasing their own type 2 diabetes drug, Mounjaro.
Within just several months of Mounjaro being released on the market for diabetes treatment, Eli Lilly petitioned the FDA for a fast track approval to market Mounjaro as an obesity drug for overweight individuals who do not have type 2 diabetes, but seek a diet drug to reduce body weight.
Eli Lilly subsequently received an FDA fast track designation for Mounjaro in October 2022, pending the results of two phase 3 trials, which were completed in April 2023.
After receiving the initial FDA approval for Mounjaro, Eli Lilly began investing hundreds of millions of dollars to enhance its production facilities and aggressively promote their new drug. In the first direct-to-consumer (DTC) ad with an estimated spend of nearly $20 million, the drug maker ran a Mounjaro commercial stating that Mounjaro can help people eat less, and that people taking Mounjaro experienced average weight reductions of up to 25 pounds.
However, as the drug gained national attention that has skyrocketed sales, the number of adverse health reports associated with Mounjaro also reached staggering numbers.
Mounjaro Side Effects Reported to the FDA
Eli Lilly now faces allegations that it placed a desire for profits before patient safety by ignoring a growing number of Mounjaro side effects reported to the FDA after the drug was released in May 2022.
According to the FDA’s FAERS database, there have been 14,733 adverse health reports linked Mounjaro as of June 30, 2023, with many involving severe gastrointestinal issues resulting in emergency room treatment.
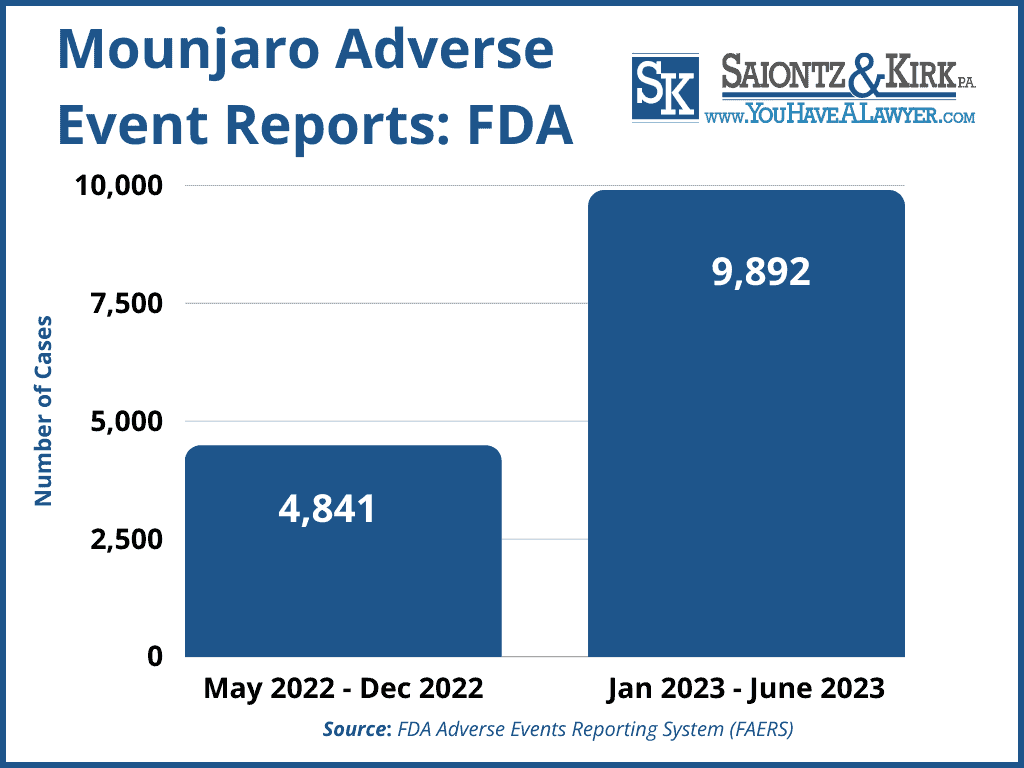
Some of the most common and severe gastrointestinal issues from Mounjaro diabetes drugs reported to the FDA have included;
- Gastrointestinal Disorder
- Gastroesophageal Reflux Disease
- Gastrointestinal Inflammation
- Impaired Gastric Emptying
- Gallbladder Disorder
- Gastric Disorder
- Gastrointestinal Hemorrhage
- Gastrointestinal Pain
- Pancreatitis
- Thyroid Cancer
- Chronic Kidney Disease
- Nausea
- Diarrhea
- Vomiting
- Abdominal Pain
- Serious Allergic Reaction
- Low Blood Sugar
Does Mounjaro cause stomach paralysis?
Similar to the allegations being raised in Ozempic Lawsuits, individuals are now pursuing legal actions against the maker of Mounjaro, alleging that the manufacturer knew about reports of stomach issues and symptoms consistent with severe gastroparesis, but failed to adequately investigate the extent or lasting nature of the side effects.
Mounjaro and Gastroparesis
Since Mounjaro is an agonist for both GLP-1 and GIP hormones of the intestines, it is believed that the drug can have compounded and synergistic impacts on the gastrointestinal systems function.
Gastroparesis arises when the stomach muscles malfunction, inhibiting their ability to adequately process and move food. This impedes the stomach from efficiently emptying itself.
Mounjaro is intentionally designed to slow gastric emptying to aid in weight loss. However, as a result of this mechanism, lawsuits claim Mounjaro causes gastroparesis and the manufacturer failed to warn that the continuous use of Mounjaro can result in prolonged and chronic delays in stomach emptying.
Symptoms of Gastroparesis from Mounjaro may include;
- Nausea
- Severe Vomiting
- Feeling full quickly when eating
- Abdominal bloating
- Stomach pain
Mounjaro Gastrointestinal Risk Studies
There is now growing evidence that Eli Lilly knew, or should have known about the gastrointestinal risks associated with Mounjaro during clinical trials. However, it appears the drug maker chose to ignore or conceal the problems, rather than accurately disclosing the full extent of the risk to potential users and the medical community.
Severe Gastrointestinal Injury Rates From Mounjaro Clinical Trial
According to an analysis of Mounjaro trials published in The New England Journal of Medicine in July 2022, individuals taking 10mg or 15mg Mounjaro injections were roughly 3x more likely to experience severe gastrointestinal events when compared to placebo.
- Placebo Group: 7 cases (1.1%)
- 10mg Mounjaro: 20 cases (3.1%)
- 15mg Mounjaro: 21 cases (3.35%)
The analysis further revealed that many Mounjaro recipients during the trials were forced to discontinue treatment due to adverse events caused by the drug. Adverse events caused treatment discontinuation in 4.3%, 7.1%, 6.2%, and 2.6% of participants receiving 5-mg, 10-mg, and 15-mg tirzepatide doses and placebo.
Mounjaro Nausea and Vomiting Side Effects
In a study published in The BMJ, dated January 29, 2024, researchers revealed a pronounced association between tirzepatide (marketed under the names Mounjaro and Zepbound) and heightened instances of gastrointestinal side effects, such as nausea, vomiting, and diarrhea.
According to the findings, tirzepatide users experienced a significant escalation in adverse gastrointestinal reactions. Specifically, the risks associated with tirzepatide, compared to placebo, included a;
- 261% greater risk of nausea,
- 392% higher chance of vomiting,
- 188% increased risk of diarrhea, and
- 130% increased likelihood of medication discontinuation due to side effects,
Mounjaro Gallbladder Risks
Researchers conducting the same study published by the New England Medical Journal further stated that individuals receiving Mounjaro during the clinical trial reported more incidents of gallbladder inflammation (Cholecystitis) than those in the placebo group.
While our lawyers are not currently pursuing Mounjaro gallbladder lawsuits, the evidence establishing this side effect provides compelling evidence that the drug maker should have recognized the Mounjaro gastroparesis side effects.
In 2017, the journal Diabetes, Obesity & Metabolism published a pivotal meta-analysis examining the effects of Ozempic and other GLP-1 receptor agonist drugs like Mounjaro. This research linked drugs like Mounjaro with an increased risk of gallbladder diseases, including gallstones and gallbladder inflammation, due to delayed gastric emptying.
When the gallbladder doesn’t regularly or fully empty, bile can accumulate, potentially raising the risk of gallstone formation. These gallstones can then obstruct the bile ducts, leading to inflammation or infection, possibly necessitating gallbladder removal.
Symptoms of gallbladder disease from Mounjaro may include;
- Intense abdominal pain
- Bloating after meals
- Indigestion or an upset stomach
- Nausea and/or vomiting
- Fever or chills
- Jaundice (yellowing of the skin and eyes)
- Dark urine and light or clay-colored stools
- Chronic diarrhea
- Belching or gas
- Intolerance to fatty foods
Our lawyers are not investigating gallbladder injury claims at this time.
Mounjaro Lawsuit Allegations
Individuals throughout the United States are currently moving forward with potential Mounjaro lawsuits against Eli Lilly and Company, raising allegations that the manufacturer,
- Failed to adequately research the link between tirzepatide and stomach problems.
- Failed to warn about the increased risk of gastrointestinal side effects
- Falsely advertised the medications as a safe weight loss drug.
- Failed to issue a Mounjaro recall to ensure consumers were aware of the health risks, or update the drug label.
Are there any costs to hire a Mounjaro lawyer?
There are absolutely no out-of-pocket costs to review your case or hire our attorneys. Mounjaro lawsuit claims are evaluated for individuals throughout the United States, and all cases are handled on a contingency fee basis.
Through the use of contingency attorney fees, individuals have access to the experience and resources of our national law firm for their Mounjaro lawsuit — regardless of their individual financial resources.
You pay nothing up front to hire our Mounjaro lawyers, and we only receive an attorney fee or expenses out of the money that is obtained from the manufacturer. Our law firm receives nothing unless we win your case!
How to File a Mounjaro Lawsuit
Complete Our Case Evaluation Request Form. Providing contact information and some information about your Mounjaro problems.
Get Contacted by Saiontz & Kirk, P.A. You will be contacted by our law firm to help determine if financial compensation may be available for you and your family.
You Decide If You Want to Move Forward. If our lawyers determine that we can help with your case then you decide whether to move forward and hire us to pursue compensation.
