Epidural Injection Meningitis Problems Could Have Been Prevented
Problems at a compounding pharmacy that distributed epidural injections nationwide, have caused an outbreak of fungal meningitis, which has already killed at least seven people and exposed hundreds of people to a risk of serious and potentially life-threatening injuries. A lack of oversight and history of regulatory issues seem to suggest that these epidural injection meningitis problems could have been preventable.
The product liability lawyers at Saiontz & Kirk, P.A are investigating potential cases for individuals throughout the United States who have been diagnosed with meningitis after receiving an epidural injection that may have been mixed by the New England Compounding Pharmacy.
▸ Request A Free Meningitis Case Evaluation
CDC Update on Fungal Meningitis Outbreak
According to updated information provided by the U.S. Centers for Disease Control and Prevention (CDC), the number of cases of fungal meningitis linked to epidural injections throughout the United States has increased from 26 confirmed cases last week, to at least 90 diagnosed cases as of Sunday.
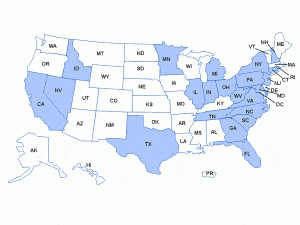 Federal and state health officials have expressed serious concerns about the scope of these problems, as the compounding pharmacy linked to the outbreak distributed more than 17,000 potentially contaminated epidural injections containing the steroid mehtylprednisolone acetate to about 75 clinics in 23 different states.
Federal and state health officials have expressed serious concerns about the scope of these problems, as the compounding pharmacy linked to the outbreak distributed more than 17,000 potentially contaminated epidural injections containing the steroid mehtylprednisolone acetate to about 75 clinics in 23 different states.
The New England Compounding Center in Massachusetts is believed to be the the cause of this nationwide and deadly meningitis outbreak. Although the pharmacy has ceased operations, the scope of this recall raises serious questions about how such massive amounts of contaminated steroid injections were able to be distributed to such a wide population so quickly.
Concerns About Compounding Pharmacy Risks and Oversight
Compounding pharmacies mix drugs in-house, supposedly on a small scale to serve local medical needs when drus are unavailable through traditional drug manufacturers. However, many are now questioning whether greed and a lack of federal oversight allowed the New England Compounding Center to grow into a “stealth” drug company, with sales agents and nationwide distribution.
The number of compounding pharmacies operating in the United States has increased from about 5,000 in 2009 to more than 7,500 three years later. They now account for $3 billion in sales and about 3% of all prescriptions filled, according to data from the International Academy of Compounding Pharmacies.
The FDA tried to regulate the industry and prevent potential compounding pharmacy problems like this several years ago, but the U.S. Supreme Court shot down rules targeting compounding pharmacies and Congress has not replaced them. Some have suggested that the FDA has more oversight over drug makers in China than it does over compounding pharmacies located in the United States.
The compounded epidural steroid injection at the center of the recent meningitis problems were not in short supply, and there was a brand name version available. However, it appears that hospitals and pain clinics ordered from New England Compounding Center because it was cheaper and had a good reputation. Unfortunately, it has taken this meningitis outbreak to raise serious questions about the role compounding pharmacies are designed to fill and the oversight provided to protect consumers.
Media reports now suggest that New England Compounding Center had less than a clean bill of health. In 2006, it appears that the FDA warned the pharmacy to stop illegal operations, including production of a standardized anesthetic topical cream, giving out drugs without a specific prescription and illegally repackaging drugs.
Despite the compounding pharmacy’s troubles, the operations at the facility continued to grow, largely unchecked. As a result of the meningitis outbreak, it appears that New England Compounding Pharmacy will face legal action from the federal government, as well as a number of personal injury lawsuits.
Meningitis Epidural Injection Lawsuits Reviewed Nationwide
Thousands of individuals throughout the United States who received an epidural injection in recent months face concerns about these contaminated steroids. In addition to contacting their treating physicians to confirm the source of their epidural injection, consumers should be aware of the signs and symptoms of meningitis, which could include:
- Dizziness
- Fever
- Nausea
- Severe Headache
Potential epidural injection meningitis lawsuits are being investigated by the lawyers at Saiontz & Kirk, P.A. for individuals throughout the United States. It appears that these problems could have, and should have, been prevented. As a result, financial compensation may be available for individuals diagnosed with meningitis following en epidural injection compounded by New England Compounding Center.
All claims for problems are reviewed by our law firm on a contingency fee basis, which means that there are no fees or expenses unless a recovery is obtained. To review a potential case for yourself, a friend or family member, request a free consultation and claim evaluation.


1 Comment • Add Your Comments
Sonya says:
I’ve suffered tremendous pain after my injections.
Posted on December 13, 2012 at 2:23 pm